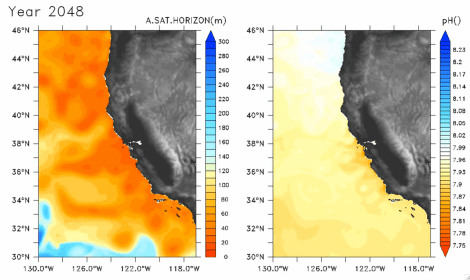
Still from an animation of projected ocean acidity.
One of the lesser-known but still widely feared effects of an atmosphere chock-full of carbon dioxide is that it makes the oceans more acidic. The process, as described by Science magazine:
About one-third of the carbon dioxide (CO2) humans pump into the atmosphere eventually diffuses into the surface layer of the ocean. There, it reacts with water to create carbonic acid and release positively charged hydrogen ions that increase the acidity of the ocean. Since preindustrial times, ocean acidity has increased by 30%. By 2100, ocean acidity is expected to rise by as much as another 150%.
The level of acidity at the surface could reach pH levels of about 7.7 by 2050. Contrary to our enticingly misleading headline, this is not nearly acidic enough to melt anyone’s toes; it’s still less acidic than distilled water. (And, besides, dissolving body parts in acid takes a long time.)
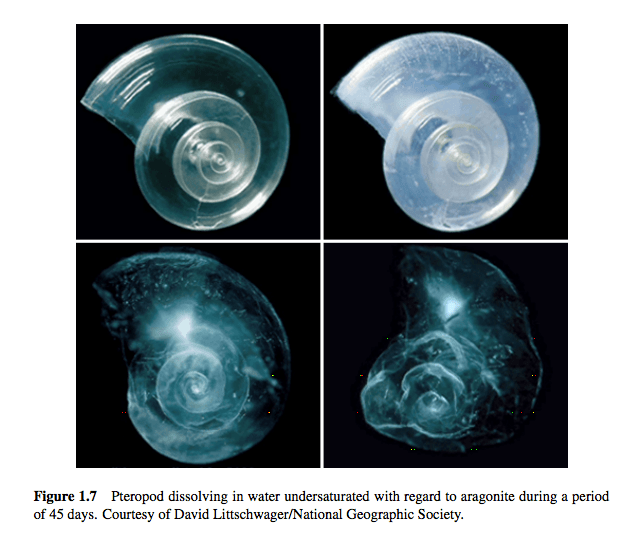
The creatures who will suffer from these chemical shifts are sea creatures. Changing the pH of the ocean changes the water’s chemistry. The more acidic the water, the fewer carbonate ions in it. The fewer carbonate ions, the less aragonite, a mineral used by organisms like oysters to build shells. At low enough levels of aragonite saturation, the shells of certain sea creatures will begin to dissolve, as illustrated below.
At the top of this post is a still from a video at the Science website, showing predicted aragonite saturation levels (left) and surface pH (right) off the coast of California in 2048. Over time, aragonite drops dramatically as swirling currents and winds mix carbon dioxide into the water.
Human surfers don’t need to worry. Oysters do.